Water Treatment Aeration

What is aeration?
Aeration is the process of bringing water and air into close contact to remove dissolved gases (e.g., carbon dioxide), oxidize dissolved metals (e.g., iron) and volatile organic chemicals (VOCs). Aeration is usually the first major process in a water treatment plant. During aeration, constituents are removed or modified before they interfere with the treatment process. An evenly distributed supply of oxygen in the aeration system is essential for effective wastewater treatment to promote microbial growth.
Aeration in water treatment
In industrial wastewater treatment, aeration is an activated sludge process in the secondary treatment process that promotes the growth of microorganisms in the wastewater. The microorganisms then feed on the organic material, creating a colony of bacteria that settles easily. Once settled in a separate settling tank, the bacteria that form the “activated sludge” population are continuously recirculated back to the aeration tank, thereby increasing the rate of decomposition.
Aeration is used in water treatment for the following operations.
- Carbon dioxide reduction (decarbonization)
- Oxidation of soluble iron and manganese to insoluble precipitates to reduce pollution
- Aeration reduces ammonia and hydrogen sulfide and is an effective method of bacteria control
Types of aerators
Aerators are divided into two main categories. They either introduce air into the water or water into the air. The water-to-air method is designed to produce small droplets of water that fall from the air. The air-to-water method produces small bubbles injected into the water stream. All aerators are designed to create more contact between air and water to enhance the transfer of gas.
Packed Tower Aerator
In this system, water falls by gravity from the top of the tower, while air is blown from the bottom of the unit in the opposite direction of the water flow. Volatile contaminants are transferred to the air by rising to the top of the tower and discharging to the outside.
Diffusion bubble aerators
A diffusion bubble aerator has several chambers and a diffuser through which air is blown. The diffuser produces fine bubbles that rise in the water as it flows from one chamber to the other. These bubbles carry volatile chemicals through the ventilation system to the outside air. The more chambers in the system, the greater the air-to-water contact.
Spray aerators
Spray aeration removes low levels of volatile contaminants, especially radon. In a spray aeration system, water enters through the top of the unit and passes through the spray nozzles in the form of a fine mist. The treated water is collected in a vented tank below the spray nozzles. Radon and other volatile pollutants are released and vented to the outside.
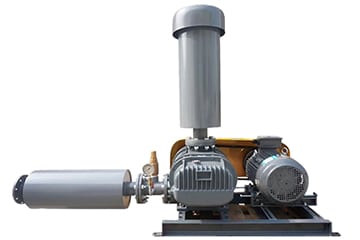
Aeration blower
The use of a certain air volume and pressure blower air or other gases through the transport equipment and diffusion equipment forced to join the water or liquid process is called air blast aeration, with this purpose of the fan is called aeration fan. The aeration process of blower is the transfer of molecular mass between gas and liquid. In order to make the gas fully diffuse and contact in the liquid and prevent the suspended matter in the liquid from sinking, the aeration fan must be able to generate enough pressure.
Roots Blower
Turbo Blower
Common operating problems
Aeration raises the dissolved oxygen content of the water. If too much oxygen is injected into the water, the water can become oversaturated, which can lead to corrosion or air binding in the filter. Other problems with aeration are slow removal of hydrogen sulfide from the tower, algae production, filter clogging and excessive energy use.
The operation of the aeration process involves the control of three basic parameters.
- Dissolved oxygen
- pH
- Temperature
The concentration of dissolved oxygen can be used to estimate whether the process is over– or under-aerated. pH testing will give an indication of the amount of carbon dioxide removed. pH increases as carbon dioxide is removed. pH can also be used to monitor the effective range of hydrogen sulfide, iron and manganese removal. Temperature is important because the oxygen saturation point increases as the temperature decreases. As the water temperature decreases, the operator must adjust the aeration process to maintain the correct dissolved oxygen level.