Electrochlorination is an increasingly popular and environmentally friendly method of water treatment that provides a sustainable way to produce chlorine-based disinfectants on-site, reducing the need for hazardous chemicals and transportation.
What is Electrochlorination?
Electrochlorination is the process of using electrical energy to convert a common salt (sodium chloride) into sodium hypochlorite, a powerful disinfectant. The technology is used in the field of water treatment to remove harmful pathogens, bacteria and viruses to ensure a safe water supply.
In simple terms, electrochlorination is like generating chlorine bleach directly from salt and water through electricity. Its chemical reaction can be expressed as:
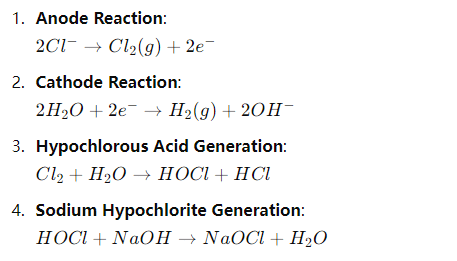
The chlorine gas (Cl₂) produced in the reaction dissolves in water and forms sodium hypochlorite (NaOCl), which acts as a disinfectant.
How does Electrochlorination Work?
This process begins by dissolving salt in water to form a brine solution. The solution is then passed through an electrolyzer where a direct current is applied. In the electrolyzer, sodium chloride (NaCl) in the brine is broken down by electrolysis into chlorine gas, hydrogen gas and sodium hydroxide.
- Chlorine gas: Dissolves in water to form hypochlorous acid (HOCl), a substance that is effective in killing microorganisms.
- Sodium hydroxide (NaOH): This is a by-product that is usually neutralized or used in other processes.
- Hydrogen: A harmless by-product that can be safely vented.
The entire system operates in a closed loop, with the chlorine produced on site being injected directly into the water system.
The principle of operation of a sodium hypochlorite generator is known as electrochlorination. The process begins by dissolving sodium chloride (table salt) in water to form a brine solution. The brine is then transported to an electrolysis cell, where it undergoes direct current electrolysis, where chlorine ions are oxidized at the anode to form chlorine gas, while water molecules are reduced at the cathode to form hydrogen gas and hydroxide ions.


The generated chlorine gas reacts with water to form hypochlorous acid (HOCl), which reacts with hydroxide ions to form sodium hypochlorite (NaOCl) under appropriate conditions. This process enables the sodium hypochlorite generator to efficiently produce sodium hypochlorite for disinfection and water treatment on site, which is widely used in the fields of drinking water, swimming pools and industrial water treatment.
Electrochlorination vs. Traditional Chlorine Disinfection Technology
| Comparison Dimension | Electrochlorination | Traditional Chlorine Disinfection |
| Technology Principle | Electrolyzes saltwater to produce sodium hypochlorite on-site | Uses chlorine gas, liquid chlorine, or sodium hypochlorite for disinfection |
| Safety | No need for transporting or storing hazardous chemicals; high safety | Requires storage and transport of chlorine gas, with potential leakage risks |
| Cost Efficiency | High initial cost, but low operating cost using salt as raw material | Lower initial cost, but long-term cost is high due to continuous chemical supply and transport |
| Environmental Impact | Low by-product generation, reduced transport needs, lower carbon emissions | Generates more harmful by-products (e.g., trihalomethanes), higher carbon footprint |
| Ease of Operation | High automation, easy maintenance | Frequent chemical replenishment needed, more complex operation |
| Application Scenarios | Ideal for large-scale water treatment plants, power plant cooling systems, desalination | Commonly used in municipal water supply, small-scale industrial water treatment |
| Energy Requirements | Requires electricity for electrolysis, relatively high energy consumption | Relies on chemicals, no extra electricity required |
| Disinfection Efficiency | High disinfection efficiency, adjustable hypochlorite concentration | High efficiency but needs regular monitoring and adjustment of chlorine levels |
| Maintenance Needs | Highly automated, low intervention required | Requires frequent equipment checks, managing chemical storage increases complexity |
| Supply Chain Dependency | Relies on common salt, stable supply chain | Dependent on external supply of chlorine gas or liquid, vulnerable to supply chain disruptions |
What are the Environmental Conditions when Electrochlorination Reacts?
During electrochlorination, several environmental conditions are critical to its performance:
- Temperature: This is usually done at ambient temperature, but higher temperatures can increase the reaction rate.
- pH: Needs to be maintained at an optimum pH (usually between 7-8) to ensure stability of the chlorine species.
- Salt concentration: A concentration of 3-5% sodium chloride is typically used to ensure that sufficient chloride ions are involved in the electrolysis.
- Electrolytic cell design: The design and material of the electrolytic cell can also affect efficiency.
Application of Electrochlorination
Electrochlorination is widely used in several industries, including:
- Municipal water treatment: Ensuring safe drinking water for communities.
- Wastewater treatment: Disinfecting treated water prior to discharge.
- Power plants: Preventing biofouling in cooling systems.
- Desalination plants: Disinfecting seawater during the desalination process to prevent microbial growth.
- Marine vessels: Treatment of ballast water to prevent the spread of alien species.
Summary
Electrochlorination is a technology that converts salt water into sodium hypochlorite through an electrolytic process. The process involves the use of direct current in an electrolytic cell to oxidize chloride ions to form chlorine gas, while water molecules are reduced to form hydrogen gas and hydroxide ions. The generated chlorine gas reacts with water to form hypochlorous acid, which in turn reacts with sodium hydroxide to form sodium hypochlorite. Electrolytic chlorination is widely used in water treatment, swimming pool disinfection and industrial water disinfection.
KUOSI offers not only sodium hypochlorite generators, but also a wide range of large-scale equipment including dosing systems, ozone generators, sludge dewatering equipment, sludge dryers, roots blowers, scrapers and wastewater screens. We are committed to providing you with comprehensive water treatment solutions, please feel free to contact us for more product information!
