In today’s industrialized society, wastewater treatment is critical to maintaining the environment and human health. Chemical wastewater treatment removes or neutralizes hazardous substances from wastewater through the use of chemicals and treatment processes that allow them to be safely discharged into the environment or recycled for other uses.
Chemical wastewater treatment and biological wastewater treatment are two different wastewater treatment methods. Chemical wastewater treatment focuses on the use of physical and chemical methods to remove pollutants from wastewater, and is particularly suitable for treating industrial wastewater, while biological wastewater treatment relies on microbial degradation of organic matter, like anaerobic wastewater treatment, and is mainly used in areas such as municipal wastewater treatment. There are significant differences between these two methods in terms of treatment principles, application areas and equipment and facilities, and the appropriate method can be selected according to the nature of the specific wastewater and treatment needs.
In this article, we will explore the importance of chemical wastewater treatment, common treatment methods and chemicals, and its positive impact on the environment.
Why is chemical wastewater treatment so important?
- Environmental protection: Chemical wastewater usually contains organics, heavy metals, hazardous chemicals and other pollutants that can cause serious pollution of water bodies, soil and ecosystems if not treated and discharged into the environment. Pollutants in wastewater can affect water quality, biodiversity and ecological balance.
- Water conservation: chemical wastewater treatment helps to conserve limited freshwater resources. By properly treating and reusing wastewater, over-exploitation of groundwater and rivers can be mitigated, ensuring a sustainable supply of water resources.
- Health and safety: Toxic substances in untreated chemical wastewater pose a threat to human health. These substances can enter the human body through drinking water, the food chain, or through direct contact, causing a variety of health problems, including cancer, poisoning, and chronic disease.
- Regulatory compliance: Most countries have implemented stringent regulations and standards that require industrial and manufacturing companies to properly treat their wastewater in order to comply with environmental regulations. Failure to comply with these regulations can result in legal liability and fines.
- Sustainability: Chemical wastewater treatment contributes to the goal of sustainable development. By reducing pollution and promoting recycling and resource reuse of wastewater, resource consumption can be reduced, energy can be conserved, and environmental impacts can be minimized.
- Resource recovery: In some cases, certain components of chemical wastewater can be recycled. For example, metal ions in wastewater can be extracted and recycled for reuse, reducing the need for virgin minerals.
- Social responsibility: Businesses and social organizations are increasingly concerned about environmental protection and social responsibility. Through effective chemical wastewater treatment, companies can demonstrate their concern for the environment and society and enhance their public image.
Common chemical wastewater treatment methods and technologies
Coagulation and flocculation
- Coagulation is the aggregation of tiny suspended particles into larger particles so that they can be more easily separated and removed. Typically, coagulants such as alum (aluminum sulfate salt) or ferric chloride are added to wastewater. These coagulants neutralize the charge on the surface of the particles, prompting attraction between the particles, which results in the formation of larger aggregates.
- Flocculation is the process of further increasing the size and mass of particles based on coagulation. Flocculants are chemicals that help form larger particles. Typically, flocculants are polymers or organic polymers that adsorb to the surface of the particles, increasing the attraction between them and thus inducing aggregation.
- Collectively, the goal of the coagulation and flocculation process is to aggregate tiny particles and solids into particles that are large and heavy enough so that they can be easily separated from the wastewater. This process usually involves mixing and agitation to ensure that the coagulation and flocculation process is fully functional.
Sediment
- Sedimentation is the process of settling larger particles that have formed into wastewater. Once the coagulation and flocculation processes have formed large and heavy enough particles, they can be separated by settling. This usually involves standing the wastewater in a container that allows the particles to sink to the bottom in the wastewater.
- Another method is flotation, in which air bubbles are passed into the wastewater to float the particles to the surface of the wastewater, where they are then removed from the wastewater. This method is particularly suitable for light particles such as grease and organic matter.
The goal of coagulation, flocculation, and sedimentation is to remove suspended solids, sludges, and suspended contaminants from wastewater in order to improve the clarity and quality of the wastewater. These steps are typically the pretreatment phase of chemical wastewater treatment and help reduce the burden on subsequent treatment processes and ensure that the wastewater meets regulatory and environmental standards.
Oxidation and reduction
Oxidation:
- Oxidation is a chemical process that involves oxidizing a contaminant into a safer or more easily removable compound. Typically, the oxidation process involves the introduction of an oxidizing agent, such as oxygen, hydrogen peroxide (also known as hydrogen peroxide), or chlorine, to induce an oxidation reaction in the contaminant.
- Oxidation is commonly used to degrade difficult-to-degrade organics such as organic dyes, solvents, and odorants. The oxidation process can break down these organic pollutants into simpler compounds or oxidize them to carbon dioxide and water.
Reduction:
- Reduction is a chemical process that involves reducing a pollutant to its lower oxidation state form. Typically, the reduction process involves the introduction of a reducing agent, such as sodium sulfite, ferrous sulfate, or hydrogen, to reduce the oxidation state of the contaminant.
- Reduction is commonly used to remove heavy metal ions such as chromium (Cr), iron (Fe), and manganese (Mn) by reducing them to a solid form that can be separated by precipitation or filtration. Reduction can also be used to reduce oxidizing odorants, thereby mitigating odor contamination.
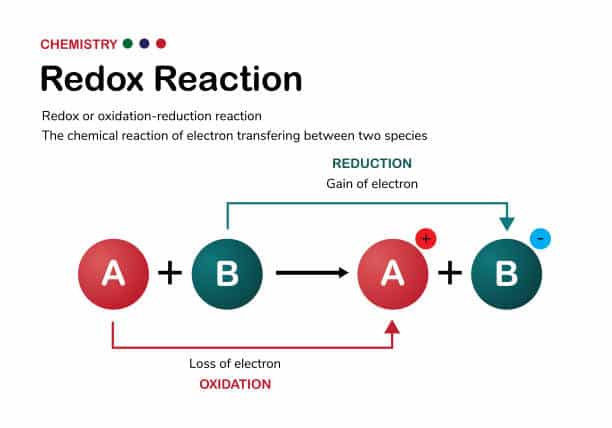
Oxidation and reduction processes are often part of advanced wastewater treatment to treat difficult-to-degrade pollutants or to reduce the concentration of specific pollutants in the wastewater. Chemical dosing systems may be used to introduce appropriate oxidizing or reducing agents to facilitate chemical reactions, such as oxidation of organic matter to harmless substances, and appropriate reaction conditions depending on the type of pollutant in the wastewater and the wastewater treatment objectives. The goal of these processes is to reduce the concentration of pollutants in the wastewater to meet regulatory requirements and to minimize their adverse environmental impacts.

Adsorption
Adsorption is an effective wastewater treatment method, especially useful for the removal of difficult-to-degrade organic pollutants. It can be used in a single wastewater treatment step or in combination with other treatment methods (e.g., precipitation, oxidation, etc.) to achieve higher wastewater purification. Selection of suitable adsorbents and optimization of adsorption conditions are important to achieve the best adsorption results.
Absorbent:
- Adsorbents are usually porous, high-surface-area solid materials, such as activated carbon, silica gel, alumina, iron oxide, zinc oxide, and so on. These adsorbents have a large number of tiny pores and highly developed surfaces, giving them excellent adsorption properties.
- Activated carbon is one of the most commonly used adsorbents, especially for the removal of organic substances because of its large number of carbon-carbon bonds on its surface, which gives it a good affinity for organic compounds.

Adsorption process:
- In the adsorption process, wastewater is brought into contact with an adsorbent and contaminants are adsorbed from the wastewater to the surface of the adsorbent. This is a physical adsorption process and is usually reversible.
- The adsorption process is usually influenced by factors such as temperature, pH, pollutant concentration, adsorption time and adsorbent properties. Optimization of these parameters can improve the adsorption efficiency.
Applications:
- Adsorption is usually used to remove organic compounds from wastewater, such as solvents, dyes, oils and fats, phenols, polycyclic aromatic hydrocarbons, and so on.
- It can also be used to remove some inorganic pollutants such as heavy metal ions, fluoride, chlorides, etc.
Regeneration:
- Once the adsorbent is saturated, the properties of the adsorbent can be restored by thermal or chemical desorption so that it can be used again. This process is often referred to as regeneration or regeneration of the adsorbent.
Ion exchange
Ion exchange is an effective wastewater treatment method, especially suitable for the removal of ionic pollutants. Selection of appropriate exchange resins and optimization of operating conditions are essential to achieve optimal wastewater purification.
Ion exchanger:
- Ion exchange typically uses solid particles as exchangers which carry exchangeable ions. These solid particles are usually polymeric resins in which the ions are directionally adsorbed on their surface. The most common ion exchange resins include anion exchange resins and cation exchange resins.
- Anion exchange resins are typically used to remove anions from wastewater, such as nitrate ions, chloride, and phosphate ions.
- Cation exchange resins are typically used to remove cations from wastewater, such as sodium, magnesium, and calcium.
Working Principle:
- In the ion exchange process, wastewater is passed through a column or bed containing an exchange resin, and ions on the ion exchange resin are exchanged with target ions in the wastewater. The target ions are adsorbed onto the resin, while the original ions on the resin are released into the wastewater.
- This process is reversible, so the exchange resin can be subjected to a regeneration cycle that washes away the original ions and restores it to a usable state.
Applications:
- Ion exchange is commonly used to remove hard water ions (calcium and magnesium ions), heavy metal ions (lead, chromium, nickel, etc.), contaminant ions (sulfate, chloride, etc.), and other ionic contaminants from wastewater.
- It is also used for deionization in wastewater to produce high purity water.
Disinfection
Disinfection is an important step in chemical wastewater treatment to kill or remove bacteria, viruses, and other microorganisms from wastewater. This process helps ensure that the wastewater is safe and does not pose a threat to the environment and public health before it is discharged or recycled.
Disinfectants:
- Disinfectants are chemicals or substances used to kill or remove microorganisms. Some commonly used disinfectants include chlorine (chlorine gas or sodium hypochlorite), ozone, ultraviolet (UV) radiation, and hydrogen peroxide.
- Chlorine is one of the most commonly used disinfectants and can be added to wastewater in either gaseous or liquid form to kill bacteria and viruses.
- Ozone is a strong oxidizing agent that can also be used for disinfection and removal of organic matter.
- Ultraviolet radiation kills microorganisms by irradiating wastewater to destroy their DNA.
- Hydrogen peroxide can be used to oxidize and remove organic matter and also has some disinfectant properties.

Working Principle:
- Disinfection works by interacting with the cellular structure or metabolic processes of microorganisms to kill or destroy them. Different disinfectants may differ in their mechanism and efficiency of disinfection.
- Disinfectants are usually added to wastewater at appropriate concentrations to ensure that disinfection is achieved without exceeding regulatory safety limits.
Applications:
- Disinfection(Ozone generator) is usually carried out at the final stage of wastewater treatment to ensure that the wastewater no longer contains active microorganisms before it is discharged into the environment or recycled for reuse.
- It is particularly suitable for situations where bacteria, viruses or other microorganisms are present in wastewater, such as municipal wastewater treatment, wastewater from healthcare facilities, and wastewater from the food industry.

Electrochemical treatment
Electrochemical reaction:
- Electrochemical treatment utilizes electrodes to perform oxidation and reduction reactions on pollutants and solutes in wastewater. These reactions typically involve an anode and a cathode, where oxidation occurs at the anode and reduction occurs at the cathode.
- Electrochemical reactions can be used to remove a wide range of pollutants, including organics, heavy metal ions, and oxidizing odorants.
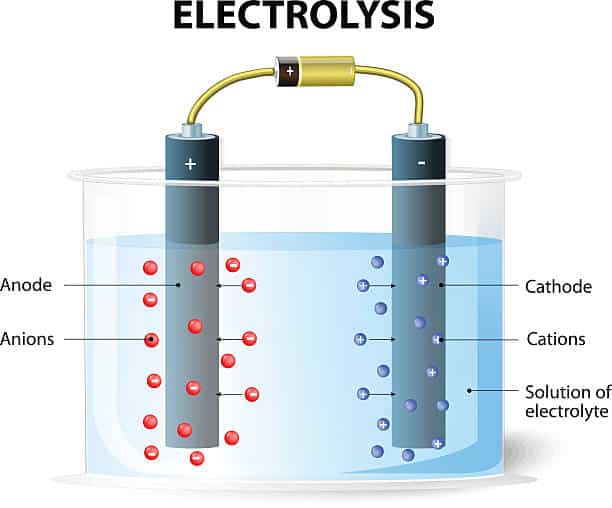
Applications:
- Electrochemical treatment can be used to treat various types of wastewater, including industrial wastewater, wastewater treatment plant outlet water, and mining wastewater.
- It is particularly suitable for the removal of difficult-to-degrade organic pollutants such as dyes, organic solvents and cyanide, as well as for the removal of heavy metal ions.
Electrochemical process:
- Common electrochemical treatment processes include electrochemical oxidation, electrochemical reduction, electrodeposition and electrodialysis.
- In electrochemical oxidation, the oxidation reaction occurs at the anode, oxidizing organic matter to carbon dioxide and water, or oxidizing heavy metal ions to their higher valence ions.
- In electrochemical reduction, the reduction reaction occurs at the cathode, reducing the organic matter to simpler compounds, or reducing the metal ions to solid metals.
- Electrochemical deposition involves the electrochemical reduction of metals from wastewater and their deposition on an electrode.
- Electrodeposition is a method of separating metal ions from wastewater that are reduced by electrochemical reactions and form deposits.
Preventive and security measures
Preventing accidents and emergencies is critical in chemical wastewater treatment. Proper storage, handling and disposal of chemicals are key to minimizing accidental spills and contamination. Employee training, monitoring systems and implementation of safety procedures are also critical steps in protecting the workplace and the environment.
Summary
Chemical wastewater treatment is a vital environmental activity aimed at the effective treatment of wastewater containing organics, heavy metals and other pollutants from a variety of industrial and domestic sources in order to purify water bodies, reduce the risk of contamination, comply with regulatory standards and, in some cases, achieve resource recovery. It involves a wide range of physical, chemical and biological treatment methods using a variety of equipment and facilities to ensure that wastewater is properly treated and disposed of in order to protect the environment and promote sustainable development.
KUOSI provides wastewater treatment equipment such as filter presses, screw presses, paddle dryers, dissolved air flotation systems, roots blowers, dosing systems, ozone generators, sodium hypochlorite generators and more. Welcome to contact us anytime.
